What is the Role of Glutamic Acid Residue in Proteins?
The role of glutamic acid residue in proteins is crucial. It is an amino acid that contributes significantly to protein structure and function. This residue plays a key role in enzyme activity and protein interactions.
glutamic acid residue helps maintain protein stability. It often engages in hydrogen bonding, which stabilizes protein folding. Additionally, it acts as a site for post-translational modifications. These modifications can alter protein activity. However, the effects of glutamic acid residues are not always positive. Misfolded proteins can lead to diseases.
Exploring the specific functions of glutamic acid residues in different proteins reveals complexity. Each protein may have unique interactions involving this amino acid. Understanding these interactions deepens our knowledge of cellular mechanisms. Yet, there remains much to learn. The nuances of glutamic acid residues and their impacts continue to challenge scientists. Further investigation is essential for a comprehensive understanding.
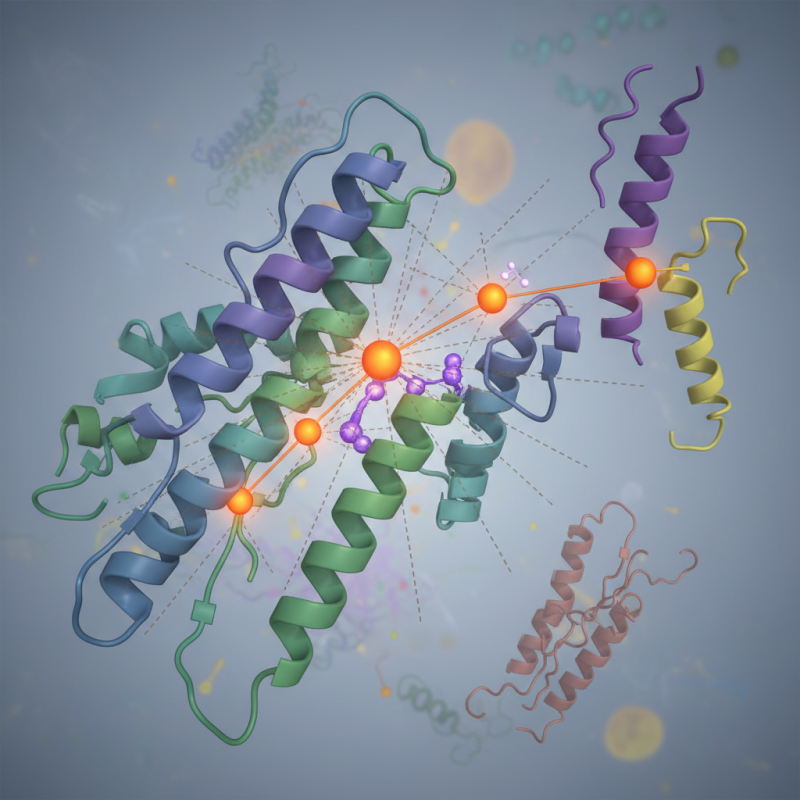
Role of Glutamic Acid Residue in Protein Structure and Stability
Glutamic acid is a vital component of many proteins. It plays a significant role in maintaining protein structure and stability. This amino acid contains a carboxyl group, giving it a negative charge at physiological pH. Such a property can lead to ionic interactions with positively charged residues, contributing to the protein's three-dimensional conformation.
Research shows that proteins rich in glutamic acid tend to exhibit enhanced stability. For instance, a study published in 2020 noted that glutamic acid residues found in the active site of enzymes assist in catalyzing reactions, which adds functional importance. However, an excess of glutamic acid can destabilize protein folding, suggesting a delicate balance is required. It raises questions about the optimization of glutamic acid in synthetic proteins.
Additionally, the presence of glutamic acid impacts protein-protein interactions. These interactions are essential for many biological processes. A report highlighted that substitutions of glutamic acid in certain proteins modified their ability to bind effectively. This highlights the nuanced role of glutamic acid in both structure and function. It begs a need for further research into its precise impacts in varied contexts. The implications are vast and require deeper exploration.
Functions of Glutamic Acid in Enzyme Catalysis and Activity
Glutamic acid plays a vital role in enzyme catalysis and activity. This amino acid is often found in the active sites of enzymes. Its side chain features a carboxylic acid group, which can donate or accept protons. This property is crucial in catalytic reactions. Enzymes often rely on glutamic acid to stabilize reaction intermediates.
In certain reactions, glutamic acid enhances substrate binding. It can interact electrostatically with positively charged substrates. This interaction can facilitate the correct orientation of the substrate for optimal catalytic action. Moreover, glutamic acid residues can participate in the transfer of protons during enzymatic processes. This allows fast and efficient catalytic cycles.
Tips: Consider the location of glutamic acid in protein structures. Its position can greatly affect enzyme efficiency. When studying enzymes, examine how changes in glutamic acid residues impact activity. Small tweaks can lead to significant differences in function. Reflect on how scientists can manipulate these residues for better enzyme performance. Understanding these aspects is essential for protein engineering.
Impact of Glutamic Acid on Protein Interactions and Binding Affinity
Glutamic acid, an amino acid with a negatively charged side chain, plays a crucial role in protein interactions. This residue, often found on protein surfaces, engages in ionic and hydrogen bonds with other molecules. Such interactions can significantly influence protein stability. For example, when glutamic acid is positioned correctly, it can facilitate effective binding with ligands. However, misplacement can lead to reduced affinity, reducing the protein's function.
The impact of glutamic acid on binding affinity is noteworthy. In enzymes, it often participates in catalysis, helping substrates fit snugly. A perfect arrangement leads to high catalytic efficiency. Conversely, if the side chain is distorted, enzyme activity may decline. This highlights the delicate balance within protein structures.
Despite its importance, not all proteins utilize glutamic acid efficiently. Some might have alternative or less effective residues in their place. This replacement often leads to complex interactions. There is still much to learn about glutamic acid's versatility and its variances among different proteins. Delving deeper may reveal new insights into protein design and function.
Post-translational Modifications of Glutamic Acid in Protein Function
Glutamic acid, an amino acid, plays a crucial role in protein function. Its carboxyl side chain allows for various post-translational modifications. These modifications can alter protein structure and activity. Phosphorylation is one such modification. It introduces a phosphate group to glutamic acid. This can enhance or inhibit enzyme activity, impacting metabolic pathways.
Another important modification is methylation. This process adds methyl groups to glutamic acid residues. Methylation can affect protein interactions and stability. Reports indicate that about 30% of proteins undergo this modification. Despite its significance, the precise effects of methylation often remain unclear. There is ongoing research exploring these interactions in depth.
Acetylation also modifies glutamic acid. This modification influences gene expression and protein interactions. Studies suggest that acetylated proteins have distinct functions. The variability in these modifications adds complexity to our understanding of protein dynamics. Researchers face challenges in mapping these changes accurately. Inconsistent data from studies hinder a comprehensive overview of glutamic acid roles. This area warrants further exploration to uncover its full impact on protein functionality.
Clinical Significance of Glutamic Acid Mutations in Diseases
Glutamic acid plays a crucial role in protein structure and function. When mutations occur in the glutamic acid residue, they can lead to serious health issues. For instance, a mutation can alter enzyme activity or disrupt the protein's stability. This can have cascading effects on metabolic pathways.
In several diseases, these mutations are significant. Take sickle cell disease as an example. Here, a single nucleotide change affects the hemoglobin protein. The resulting protein has a different charge due to altered glutamic acid residues. This change affects how hemoglobin behaves in red blood cells.
Not all effects are well understood. Research is ongoing, and every finding leads to new questions. Glutamic acid mutations may contribute to neurodegenerative diseases too. Their role is complex and not entirely clear yet. Unraveling this could change therapeutic approaches in the future.